View SELECT primary and key secondary endpoint data
EXPLORATORY POST HOC ANALYSIS FROM THE SELECT TRIAL
LENVIMA in patients with BRAF mutation+ status
Baseline patient histology in the SELECT trial
182 samples were analyzed for BRAF mutation status1
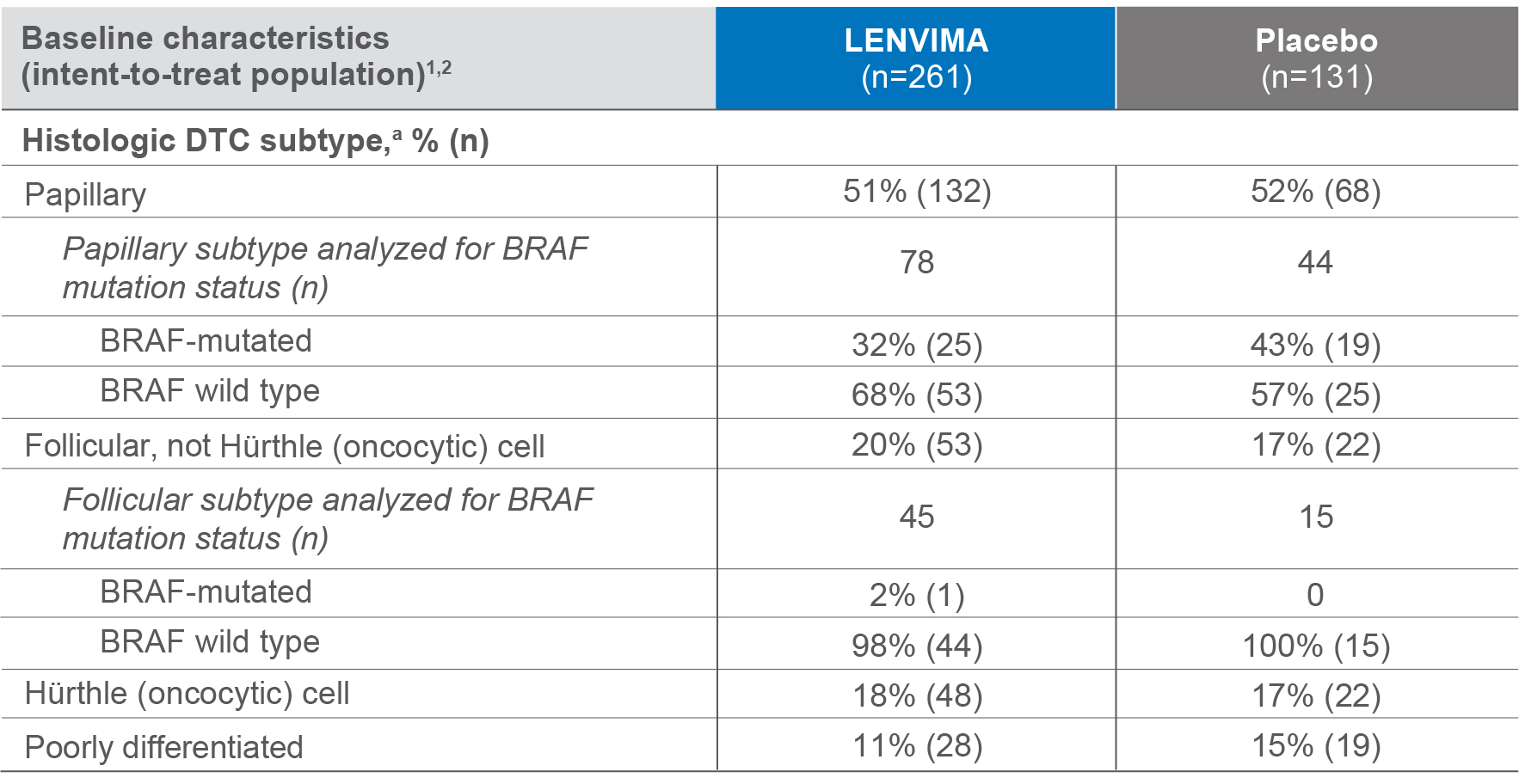
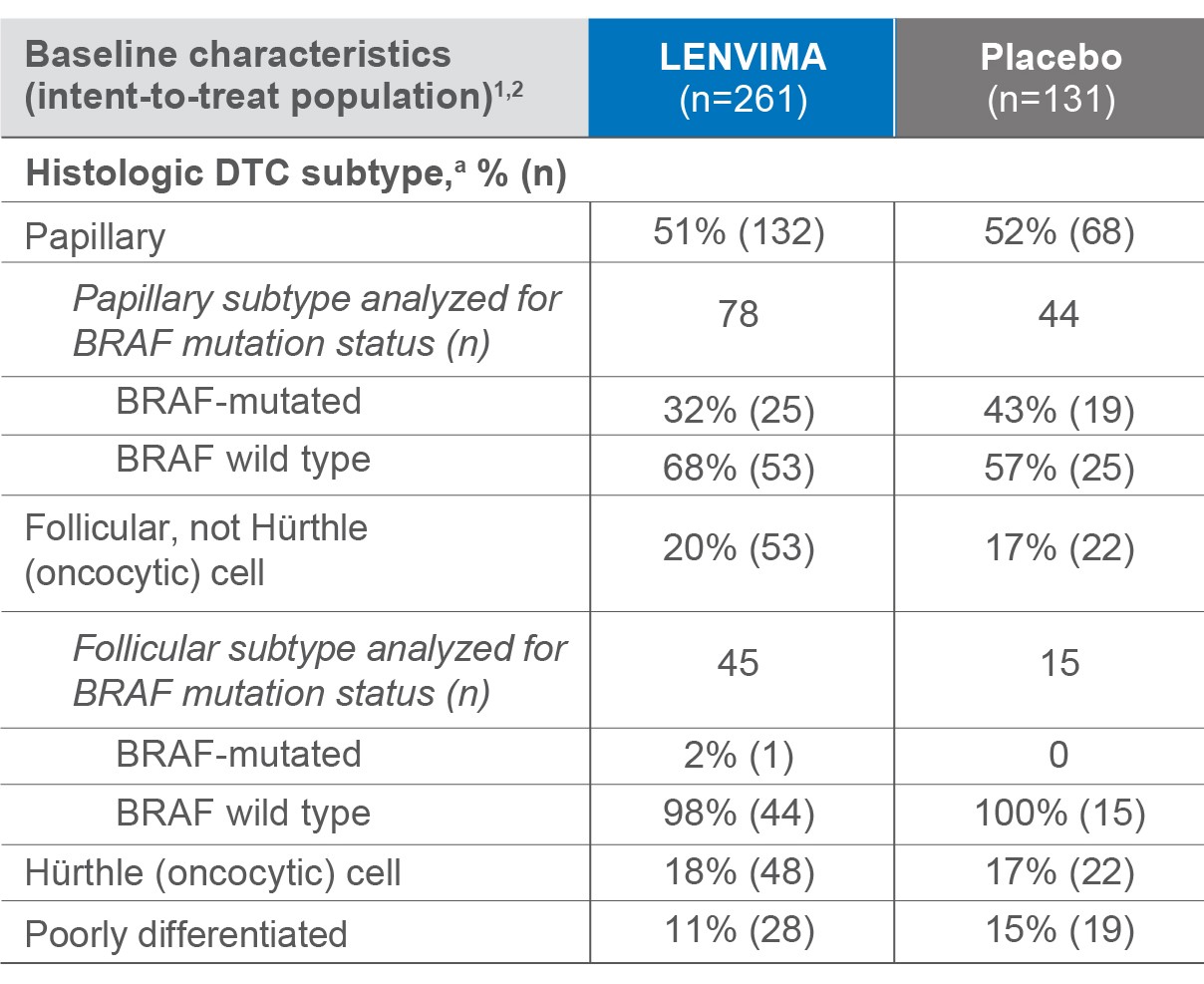
a Histologic findings were determined from investigators' reports.
•The median cumulative RAI activity administered prior to study entry was 350 mCi (12.95 GBq)3
SELECT data cutoff: November 15, 2013.
SELECT=Study of (E7080) LEnvatinib in Differentiated Cancer of the Thyroid; DTC=differentiated thyroid cancer; RAI=radioactive iodine; mCI=millicurie; GBq=gigabecquerel.
-
View PFS across certain exploratory subgroups
Forest plot of hazard ratios for PFS in certain exploratory subgroups2
SELECT data cutoff: November 15, 2013.
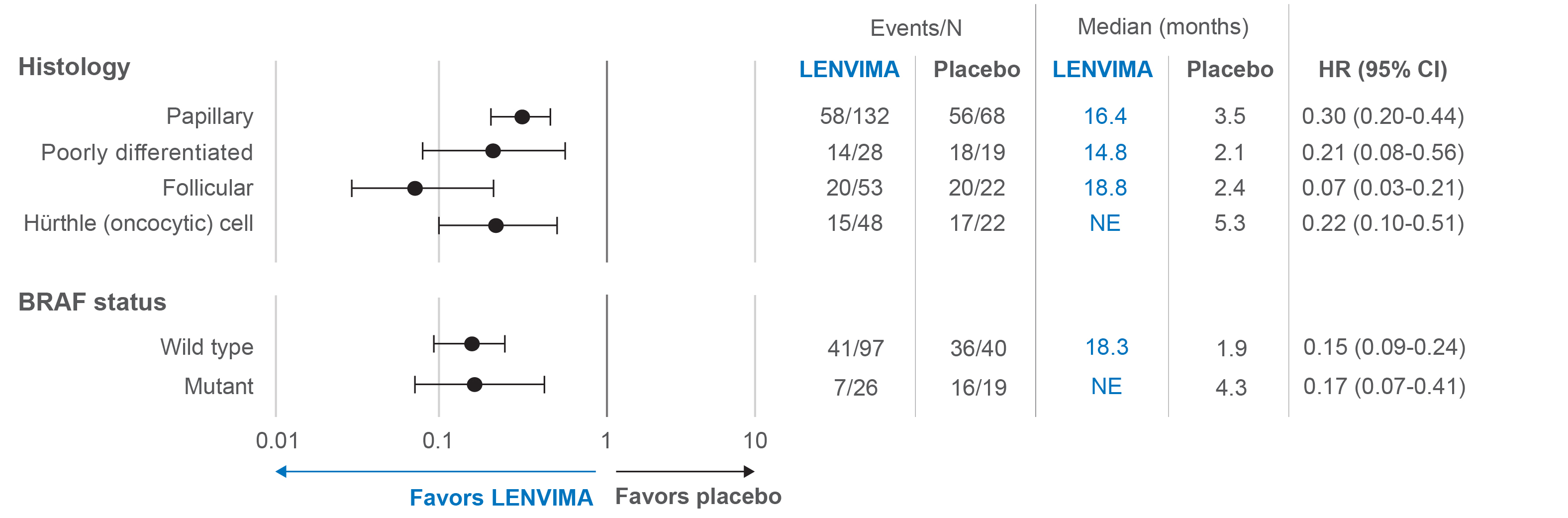
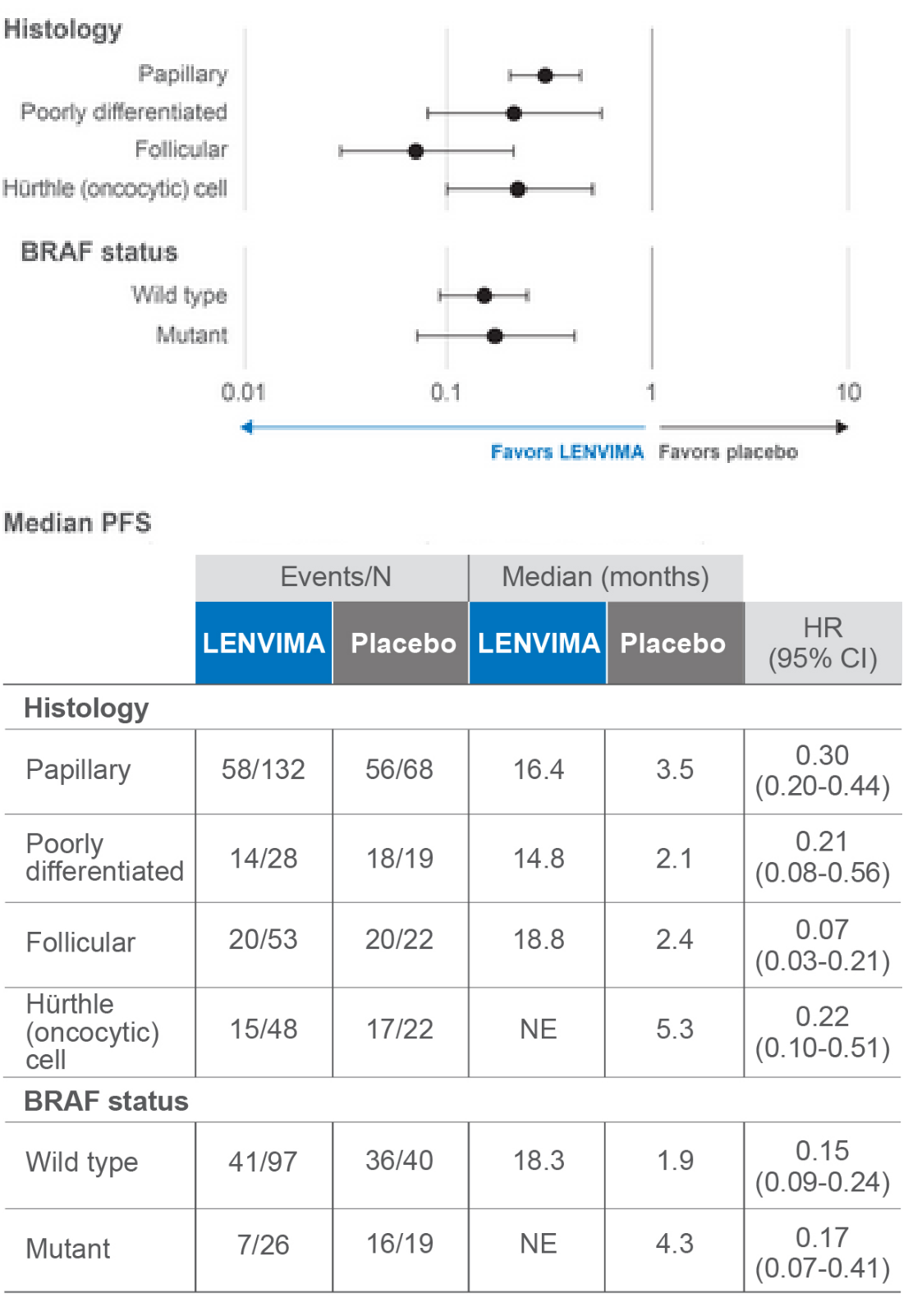
Limitations: The SELECT trial was not powered to detect differences in the treatment effect in these subgroups. No statistical testing was planned for this subgroup analysis and no adjustment for multiplicity was made, therefore, no conclusions can be drawn.
HR=hazard ratio; PFS=progression-free survival; SELECT=Study of (E7080) LEnvatinib in Differentiated Cancer of the Thyroid; CI=confidence interval; NE=not estimable.
Exploratory post hoc analysis of papillary thyroid cancer cohort from SELECT (n=122)1
SELECT post hoc exploratory analysis data cutoff: September 1, 2016.
Progression-Free Survival (PFS) by BRAF status1
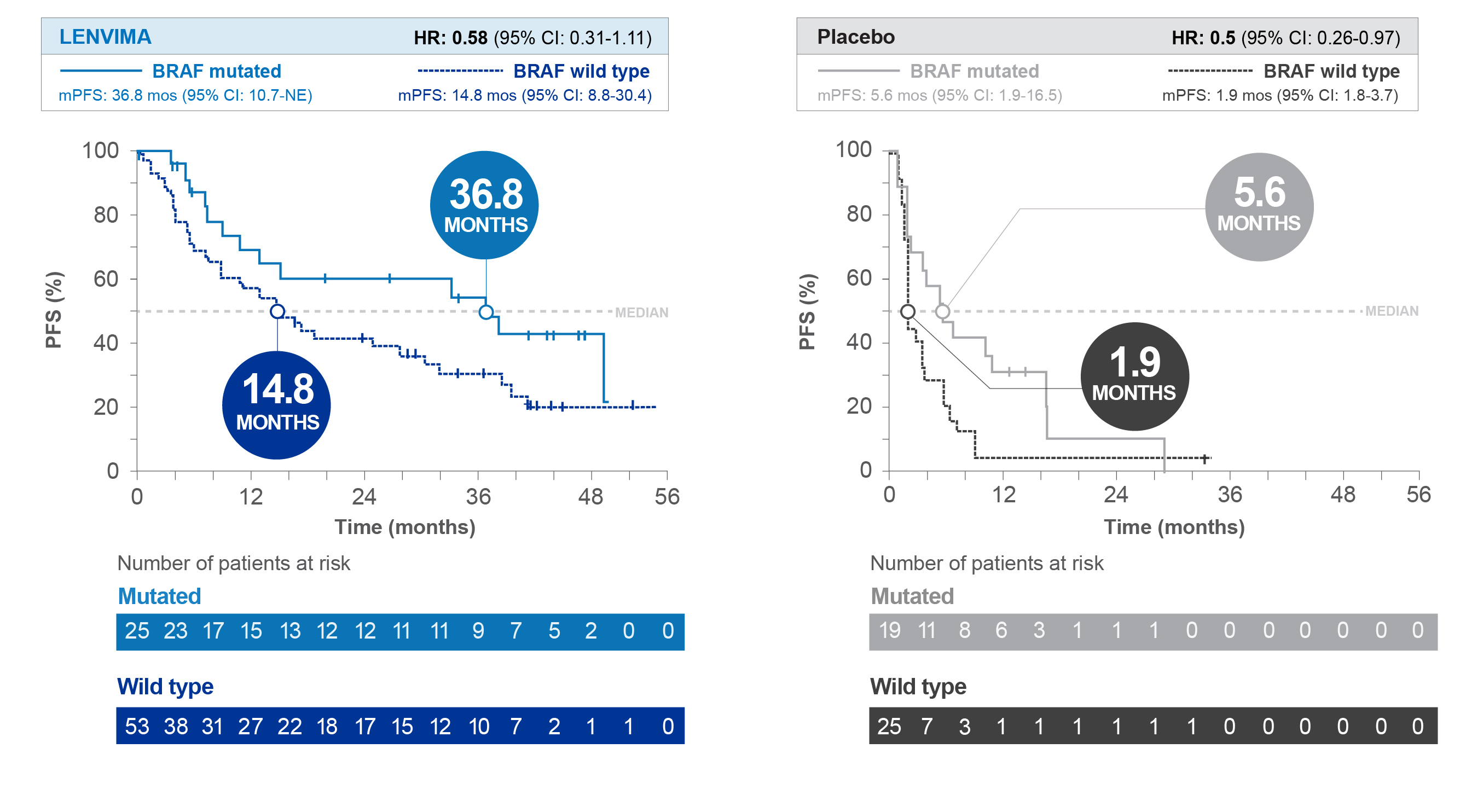
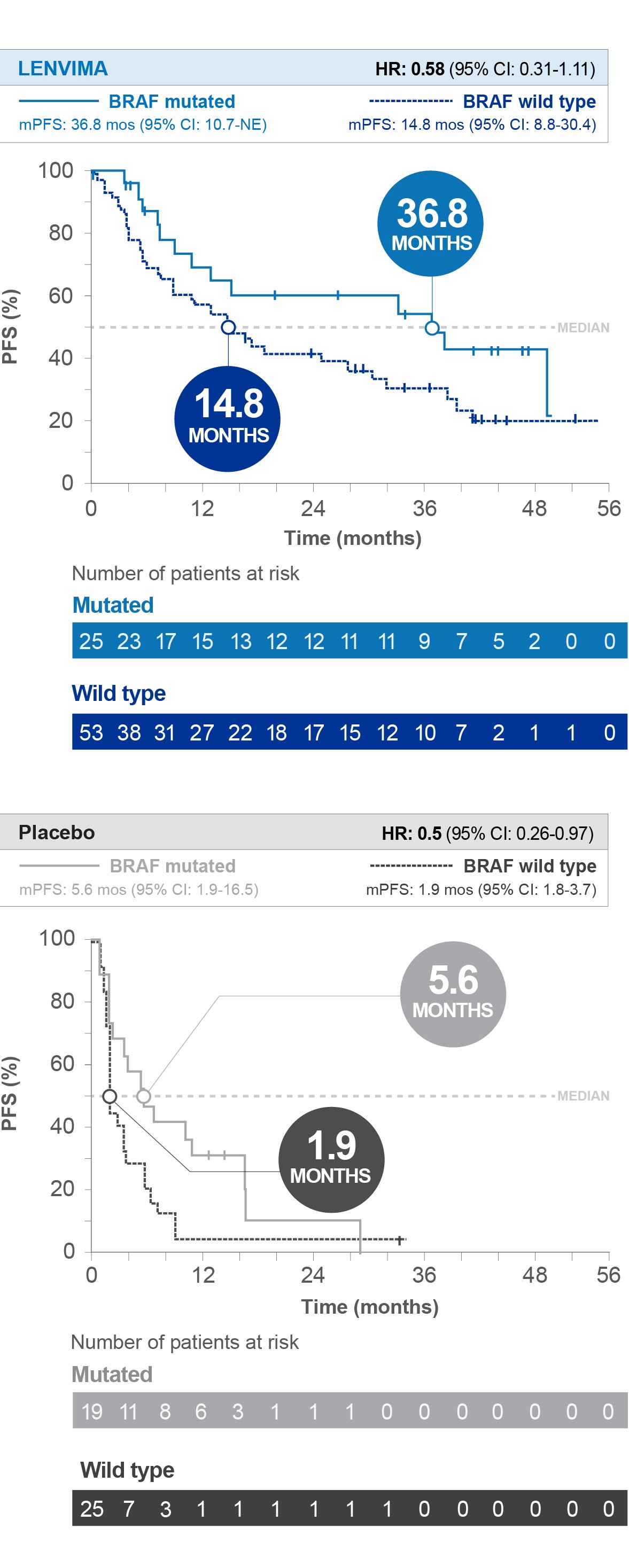
Limitations: Biomarker analyses are exploratory, and results are hypothesis-generating. The post hoc subgroup analysis was not a prespecified study endpoint, and the outcomes cannot be compared across treatment groups. No conclusions can be drawn. Given the sample size, results should be interpreted with caution. Data are descriptive only.
SELECT=Study of (E7080) LEnvatinib in Differentiated Cancer of the Thyroid; PFS=progression-free survival; HR=hazard ratio; CI=confidence interval; mPFS=median progression-free survival; NE=not estimable; ORR=objective response rate; OS=overall survival.
Objective Response Rate (ORR) by BRAF status1
•52.0% (95% CI: 31.3%-72.2%; n=13/25) in patients with BRAF mutation+ status vs 64.2% (95% CI: 49.8%-76.9%; n=34/53) in patients with BRAF wild-type status with LENVIMA
•5.3% (95% CI: 0.1%-26.0%; n=1/19) in patients with BRAF mutation+ status vs 4.0% (95% CI: 0.1%-20.4%; n=1/25) in patients with BRAF wild-type status with placebo
SELECT post hoc exploratory analysis data cutoff: September 1, 2016.
CI=confidence interval; SELECT=Study of (E7080) LEnvatinib in Differentiated Cancer of the Thyroid.
Overall Survival (OS) by BRAF status1
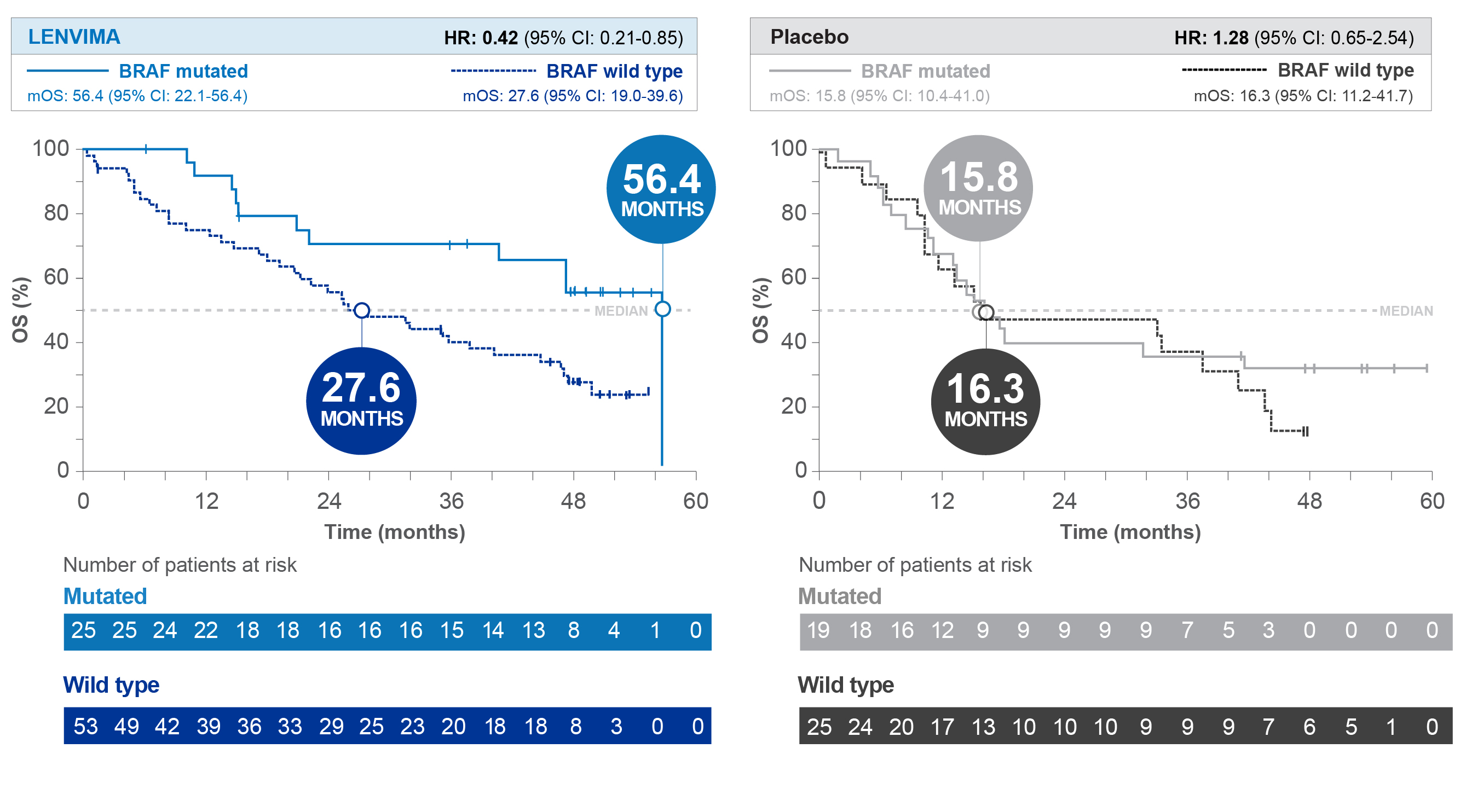
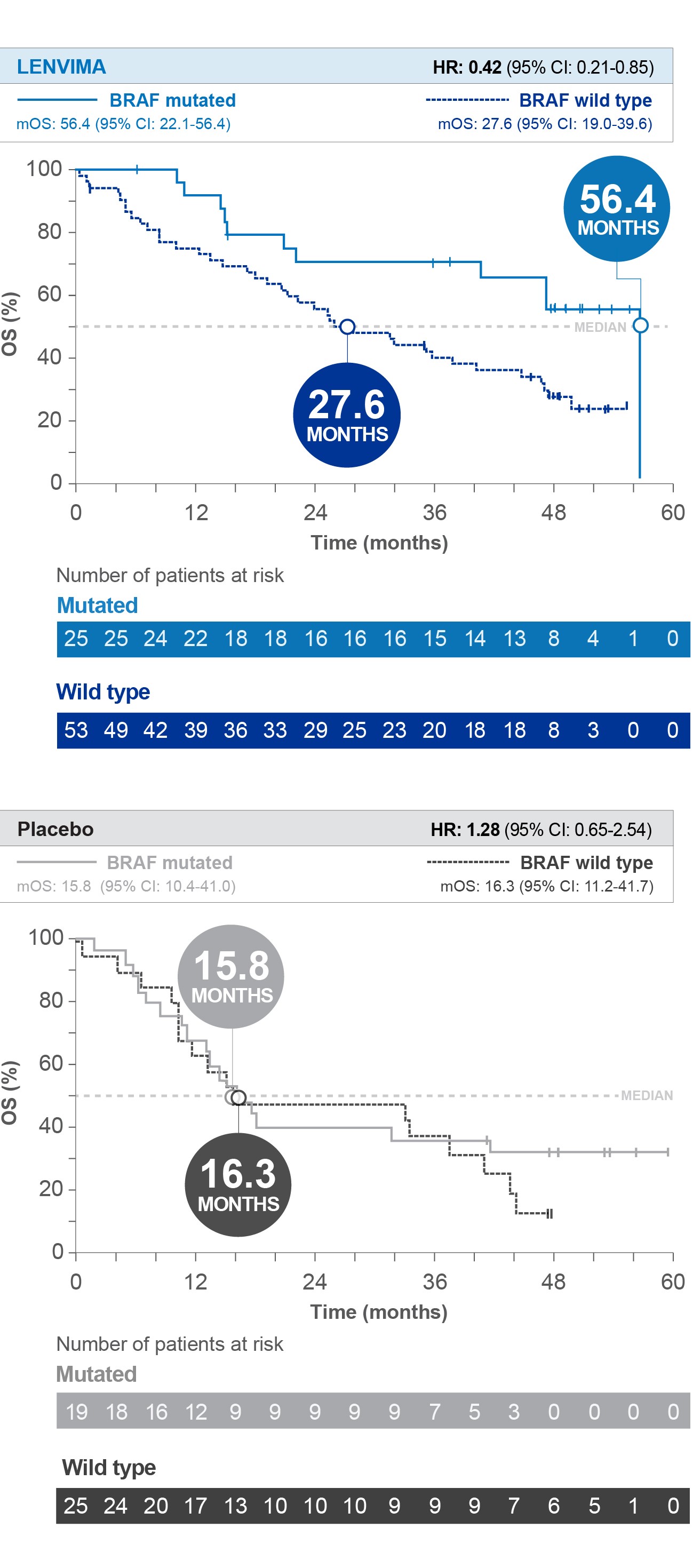
SELECT post hoc exploratory analysis data cutoff: September 1, 2016.
HR=hazard ratio; CI=confidence interval; mOS=median overall survival; SELECT=Study of (E7080) LEnvatinib in Differentiated Cancer of the Thyroid.
Adverse events by BRAF status4
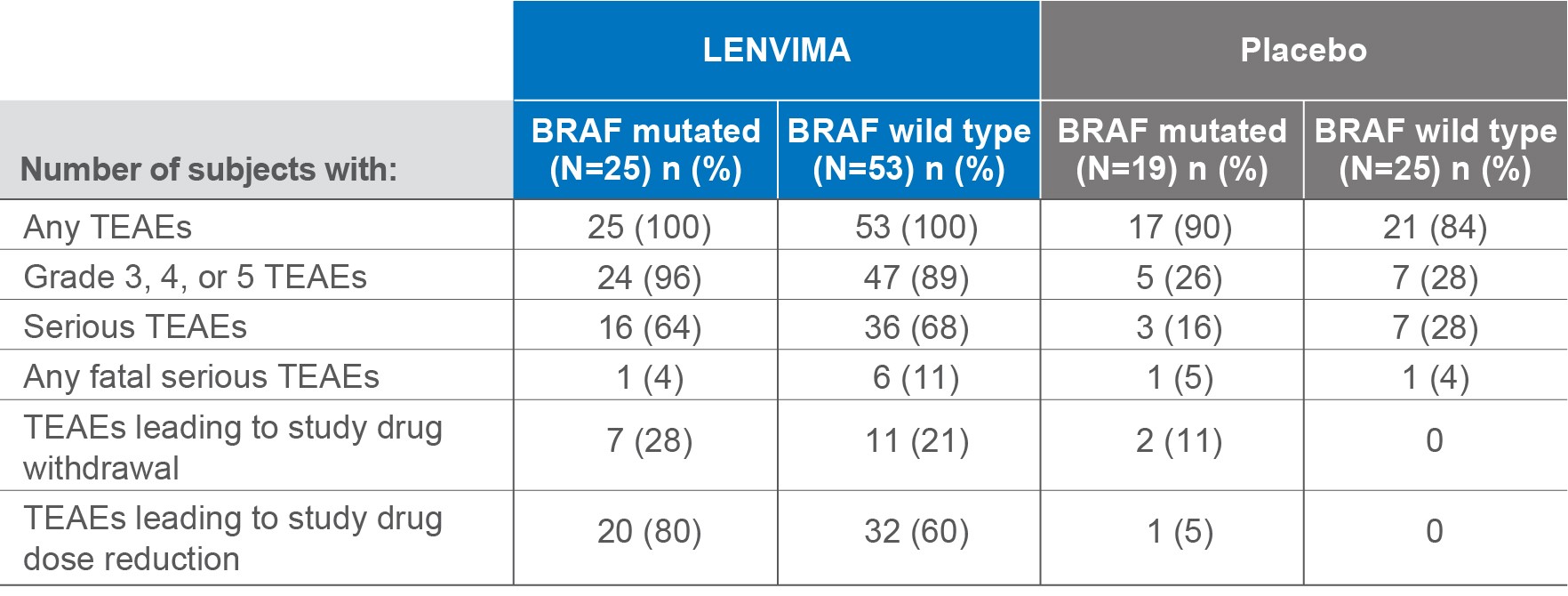
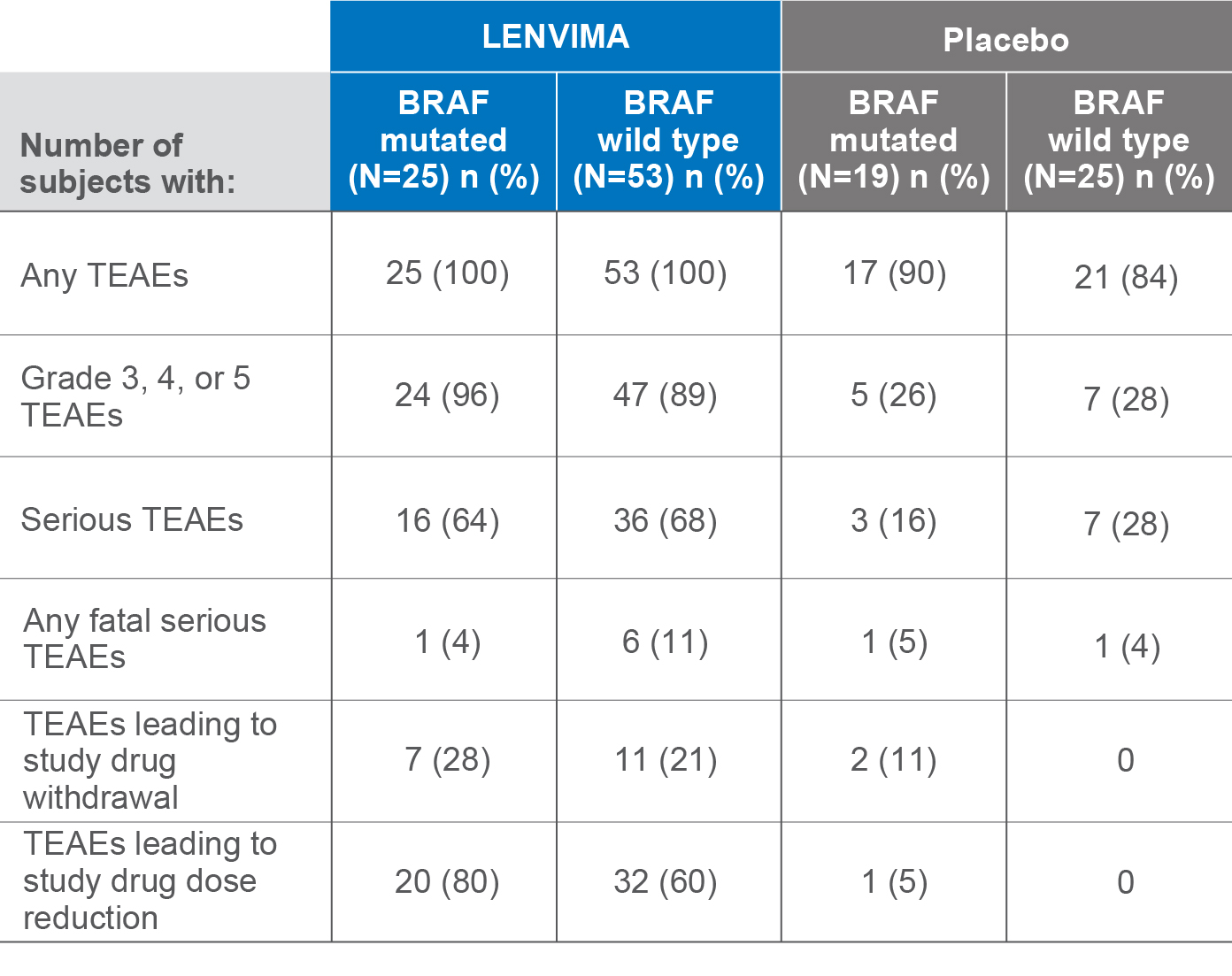
SELECT post hoc exploratory analysis data cutoff: September 1, 2016.
TEAEs=treatment-emergent adverse events; SELECT=Study of (E7080) LEnvatinib in Differentiated Cancer of the Thyroid.
