View SELECT primary and key secondary endpoint data
A real-world evidence study of first-line (1L) LENVIMA monotherapy in RAI-R DTC in the United States1
Objective
• To describe the real-world treatment patterns and clinical outcomes in patients with RAI-R DTC treated with 1L LENVIMA monotherapy in the United States*
Study Design
• A retrospective patient medical chart review was conducted. Data from 308 adult patients who initiated LENVIMA monotherapy for RAI-R DTC in 1L between February 13, 2015 and September 30, 2020 were included
• Data were extracted from patient charts by physicians from academic and community practices across the United States. Up to a maximum of 3 physicians from the same practice were allowed to participate. Each physician was allowed to provide data for ≈5 randomly selected eligible patients
• The study was approved by an institutional review board (IRB)
• All patient data were de-identified and compliant with Health Insurance Portability and Accountability Act (HIPAA) guidelines
Clinical Outcomes
• Clinical outcomes assessed included real-world best overall response (rwBOR), real-world progression-free survival (rwPFS), and overall survival (OS), as reported by physicians, based on patient medical records
SELECT=Study of (E7080) LEnvatinib in Differentiated Cancer of the Thyroid; RAI-R=radioactive iodine-refractory; DTC=differentiated thyroid cancer.
* LENVIMA is indicated for the treatment of adult patients with locally recurrent or metastatic, progressive, radioactive iodine-refractory differentiated thyroid cancer (RAI-R DTC).2
Select patient demographics and baseline characteristics1,3
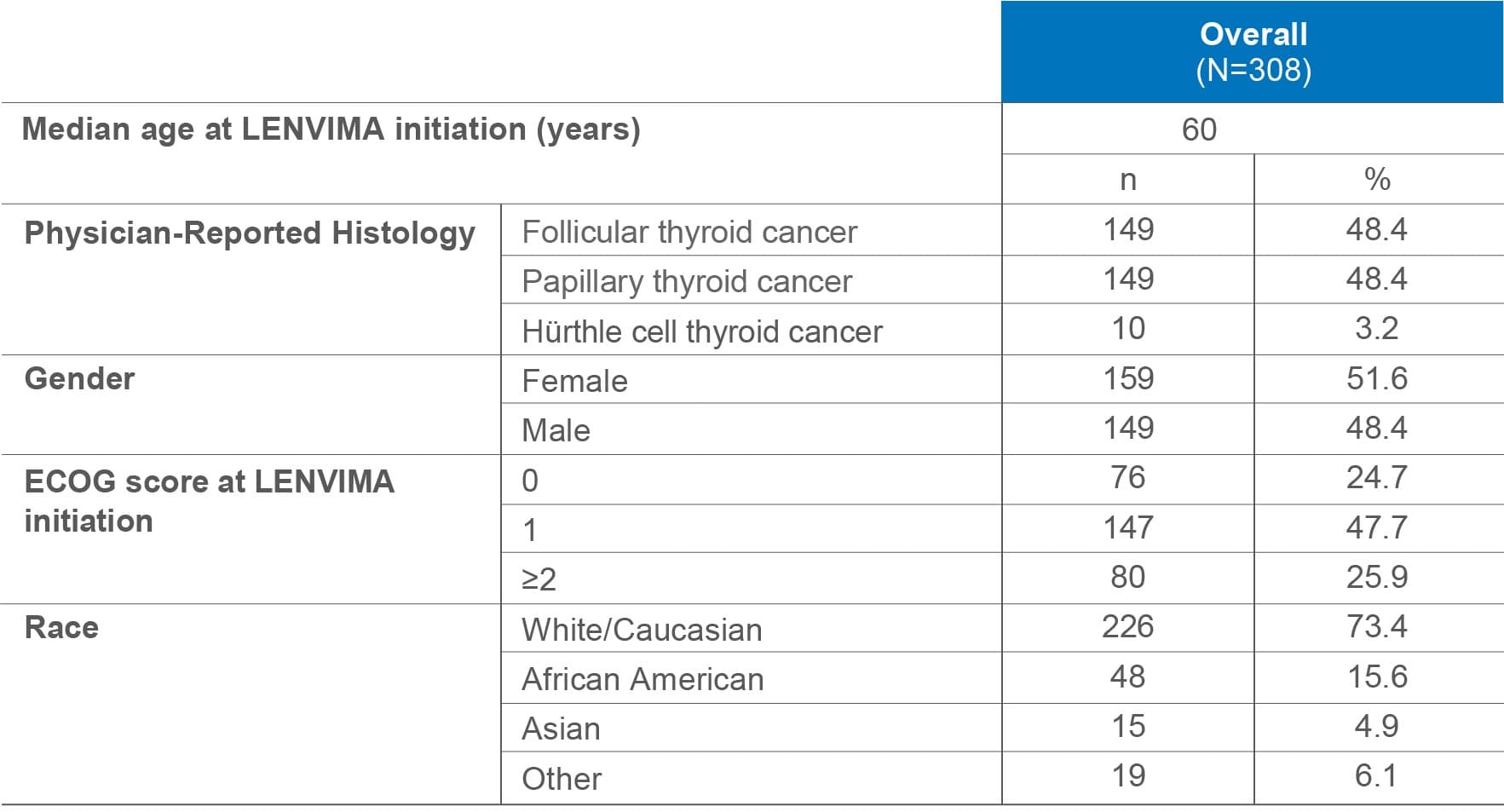
ECOG=Eastern Cooperative Oncology Group.
Sites of metastases included lymph nodes (42.9%), lung (33.1%), bone (20.1%), liver (15.3%), brain (4.2%), breast (2.9%), and skin (1.3%).3
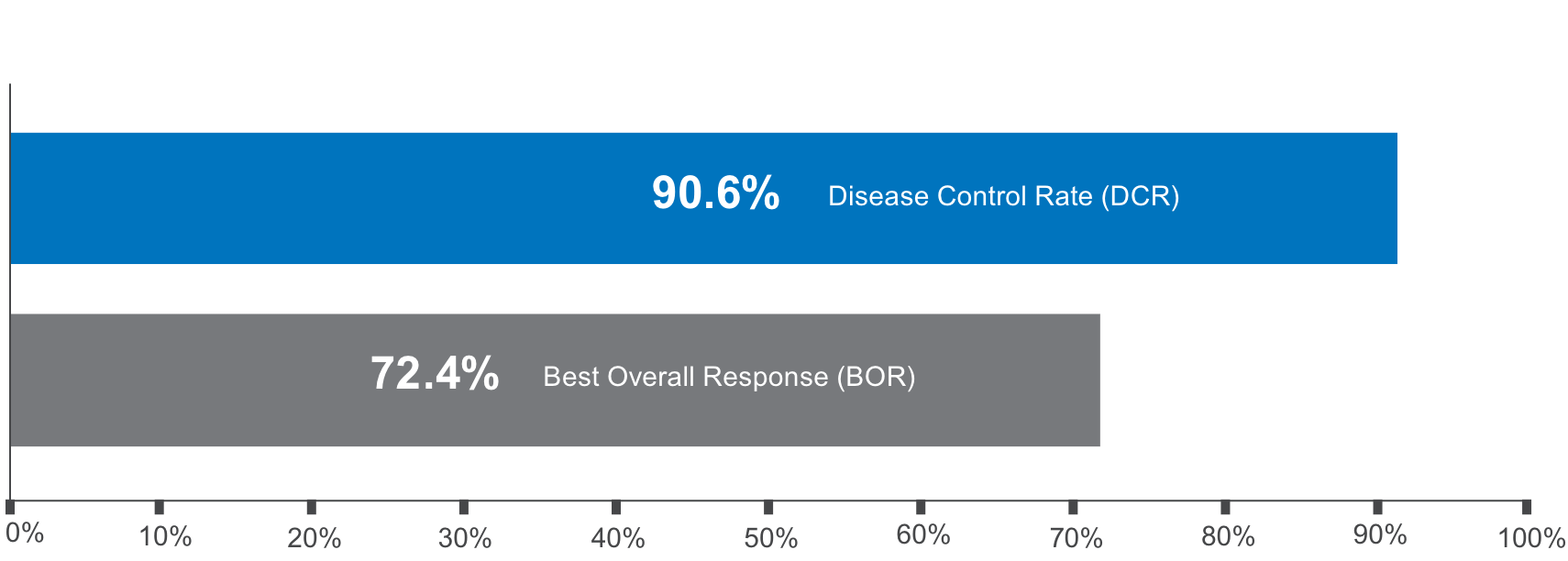
Physician-reported real-world DCR and BOR1-4
RAI-R=radioactive iodine-refractory; DTC=differentiated thyroid cancer; rwDCR=real-world disease control rate; CR=complete response; PR=partial response; SD=stable disease; rwBOR=real-world best overall response; RECIST v1.1=Response Evaluation Criteria in Solid Tumors version 1.1; iRECIST=immune Response Evaluation Criteria in Solid Tumors; rwCR=real-world complete response; rwPR=real-world partial response; rwSD=real-world stable disease; rwPD=real-world progressive disease; MRI=magnetic resonance imaging; PET=positron emission tomography; CT=computed tomography; MRS=magnetic resonance spectrometry; SELECT=Study of (E7080) LEnvatinib in Differentiated Cancer of the Thyroid; PD=progressive disease; CI=confidence interval.
Limitations: No direct comparisons between results from the pivotal clinical trial and the real-world data study should be made, as there could be potential differences in patient populations and differences in real-world clinical practices versus clinical trials. This is a single cohort, retrospective, non-interventional study with limited follow-up time and could be subject to potential provider selection bias as provider consent was required. Information on adverse events was not collected in this study.
Limitations apply to all data shown below, including real-world PFS, OS, and treatment patterns.
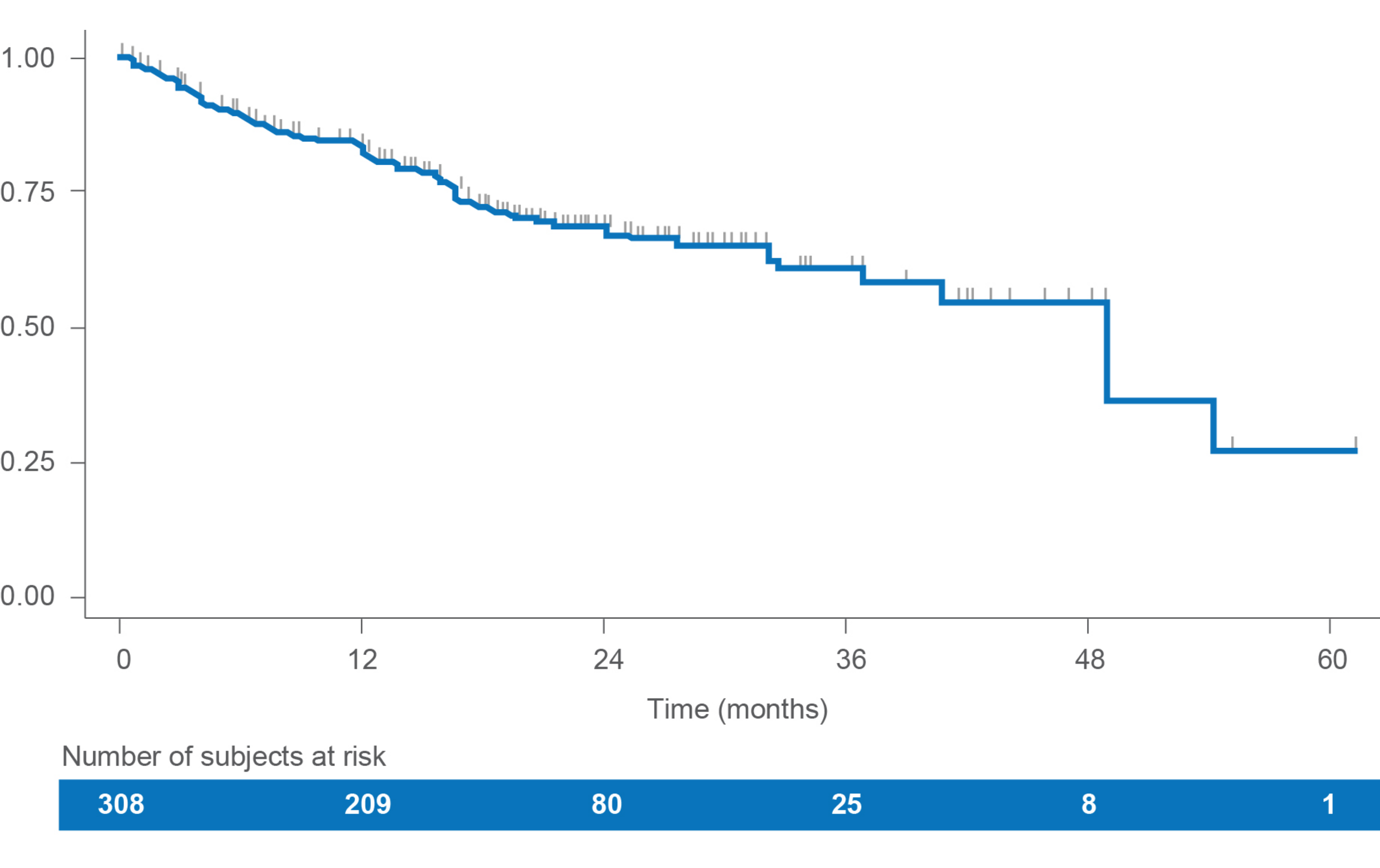
Progression-free survival (PFS)
In this retrospective study of LENVIMA monotherapy in patients with RAI-R DTC (N=308), median rwPFS was 49.0 months (95% Cl: 37.0-NE).1
• Estimated PFS rates were:
– 83.3% (95% Cl: 78.3-87.2) at 12 months
– 68.5% (95% Cl: 62.0-74.1) at 24 months
• The median time of follow-up was 18.9 months (IQR: 12.3-24.7)
PFS1

LENVIMA clinical trial results (SELECT)
• 18.3 months median PFS (95% CI: 15.1-NE) with LENVIMA vs 3.6 months (95% CI: 2.2-3.7) with placebo (HR: 0.21 [95% CI: 0.16-0.28]); P<0.0012
RAI-R=radioactive iodine-refractory; DTC=differentiated thyroid cancer; rwPFS=real-world progression-free survival; CI=confidence interval; NE=not estimable; SELECT=Study of (E7080) LEnvatinib in Differentiated Cancer of the Thyroid.
Overall survival (OS)
In this retrospective real-world study of LENVIMA monotherapy in patients with RAI-R DTC, median OS was not reached over the available follow-up period.1
OS1
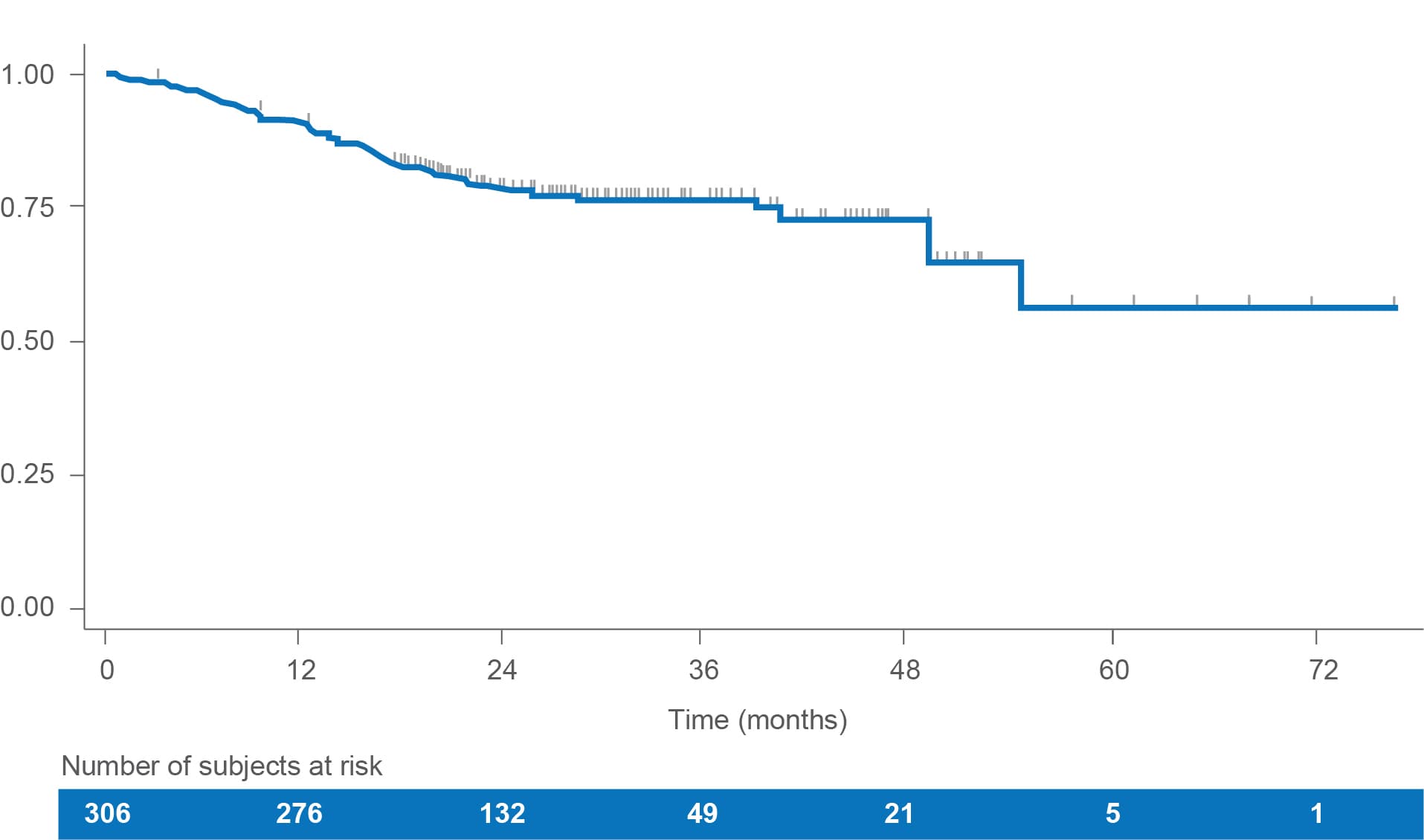
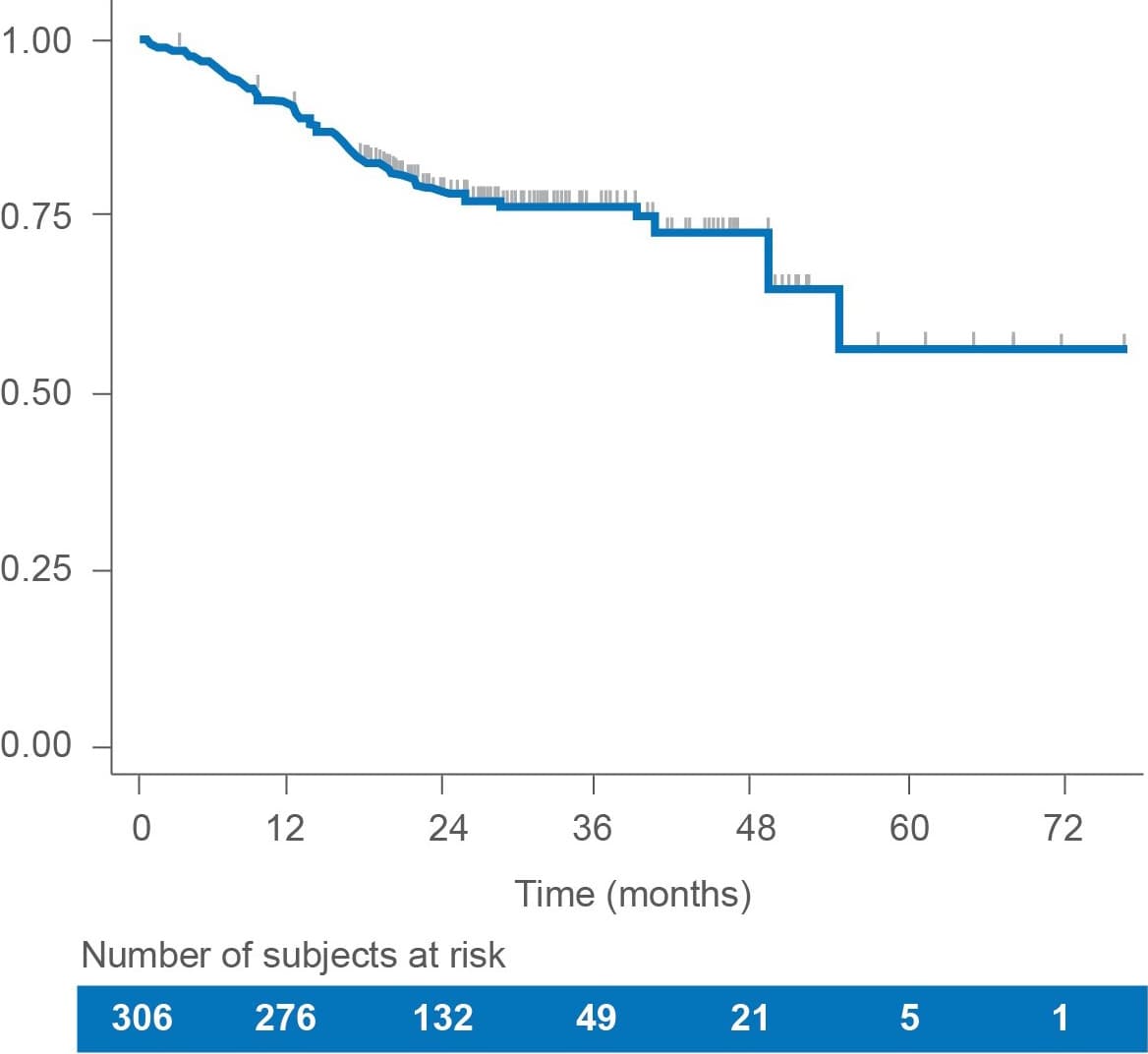
• Estimated OS rates were1:
-
– 90.8% (95% CI: 87.0-93.6) at 12 months
-
– 78.4% (95% CI: 73.0-82.8) at 24 months
• At the end of the follow-up, 75% of patients were still alive
LENVIMA clinical trial results (SELECT)
• Median OS was not estimable at data cutoff (HR: 0.73 [95% CI: 0.50-1.07]; P=0.10)2
• Kaplan-Meier estimates were*
-
– 12-month OS rate, % (95% CI): 81.6 (76.2-85.8) for LENVIMA4
-
– 24-month OS rate, % (95% CI): 58.2 (46.0-68.6) for LENVIMA4
RAI-R=radioactive iodine-refractory; DTC=differentiated thyroid cancer; SELECT=Study of (E7080) LEnvatinib in Differentiated Cancer of the Thyroid; CI=confidence interval.
* OS was adjusted for potential crossover with the use of rank-preserving structural failure time (RPSFT) model.4
LENVIMA treatment patterns1


LENVIMA real-world treatment patterns
• Among the 100 patients who discontinued LENVIMA, the most common reasons were disease progression (38.0%) and death (33.0%)
• Among patients who discontinued LENVIMA, 19 initiated a second-line treatment
By descriptive analysis, median duration of LENVIMA treatment at end of follow-up was 17.5 months (IQR: 8.8-25.0) for the overall population. Among those who discontinued LENVIMA, median duration was 9.1 months (IQR: 5.1-16.1). Among those still receiving LENVIMA, median duration was 20.1 months (IQR: 15.9-27.8).
62% of patients initiated LENVIMA at the recommended 24 mg daily dose; the remaining patients initiated at 14 mg to 20 mg daily doses. Approximately 8% of patients required a dose alteration during LENVIMA treatment.
IQR=interquartile range.