Help your patients along the LENVIMA treatment journey:
Understand possible ARs that may occur
Explore a post hoc analysis of time to onset of select ARs
Most common serious ARs (≥2%) in the LENVIMA arm1
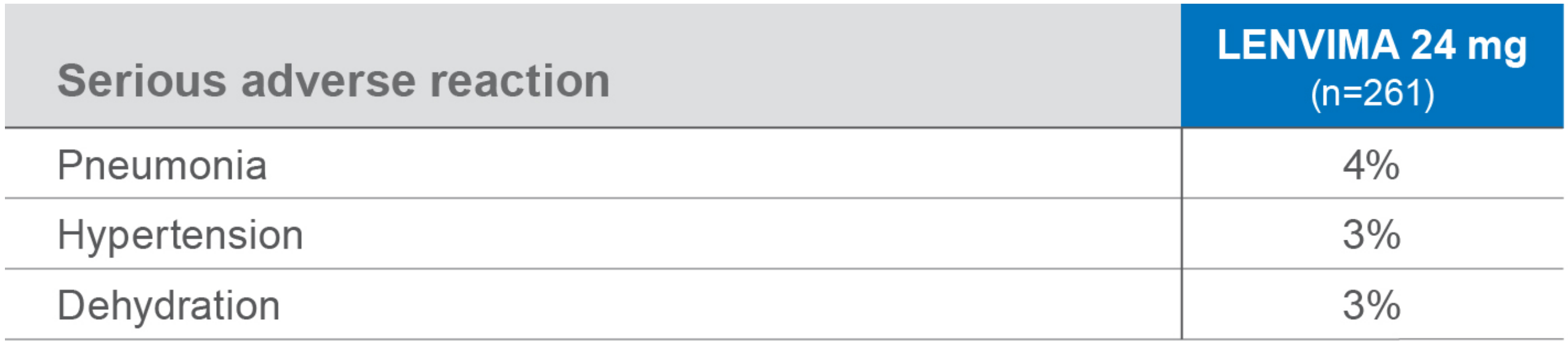
ARs=adverse reactions.
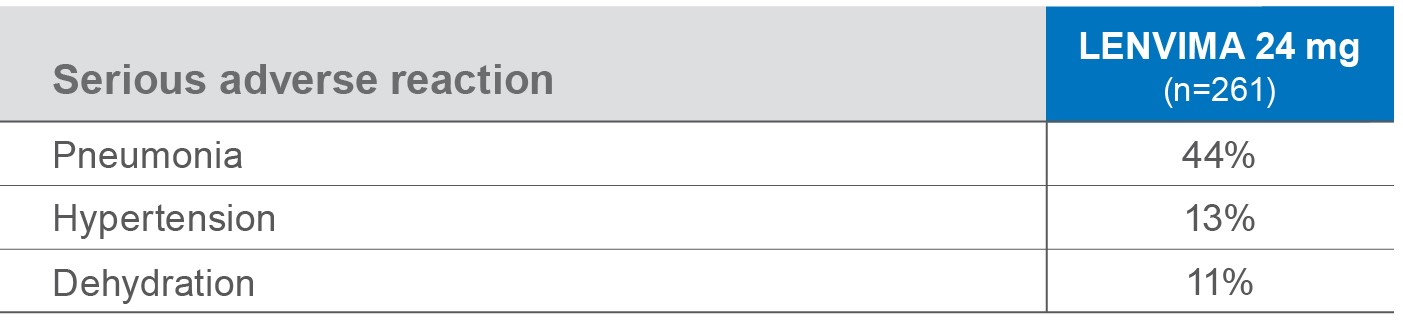
Most common Grade 3-4 ARs (≥5%)1

No Grade 4 diarrhea, hand-foot skin reaction, fatigue, or proteinuria were reported.2
- aIncludes hypertension, hypertensive crisis, increased blood pressure diastolic, and increased blood pressure.
- bIncludes asthenia, fatigue, and malaise.
- cIncludes musculoskeletal pain, back pain, pain in extremity, arthralgia, and myalgia.
- dIncludes aphthous stomatitis, stomatitis, glossitis, mouth ulceration, and mucosal inflammation.
ARs=adverse reactions.
ARs occurring in patients with a between-group difference of ≥5% (all grades) or ≥2% (Grade 3-4)1

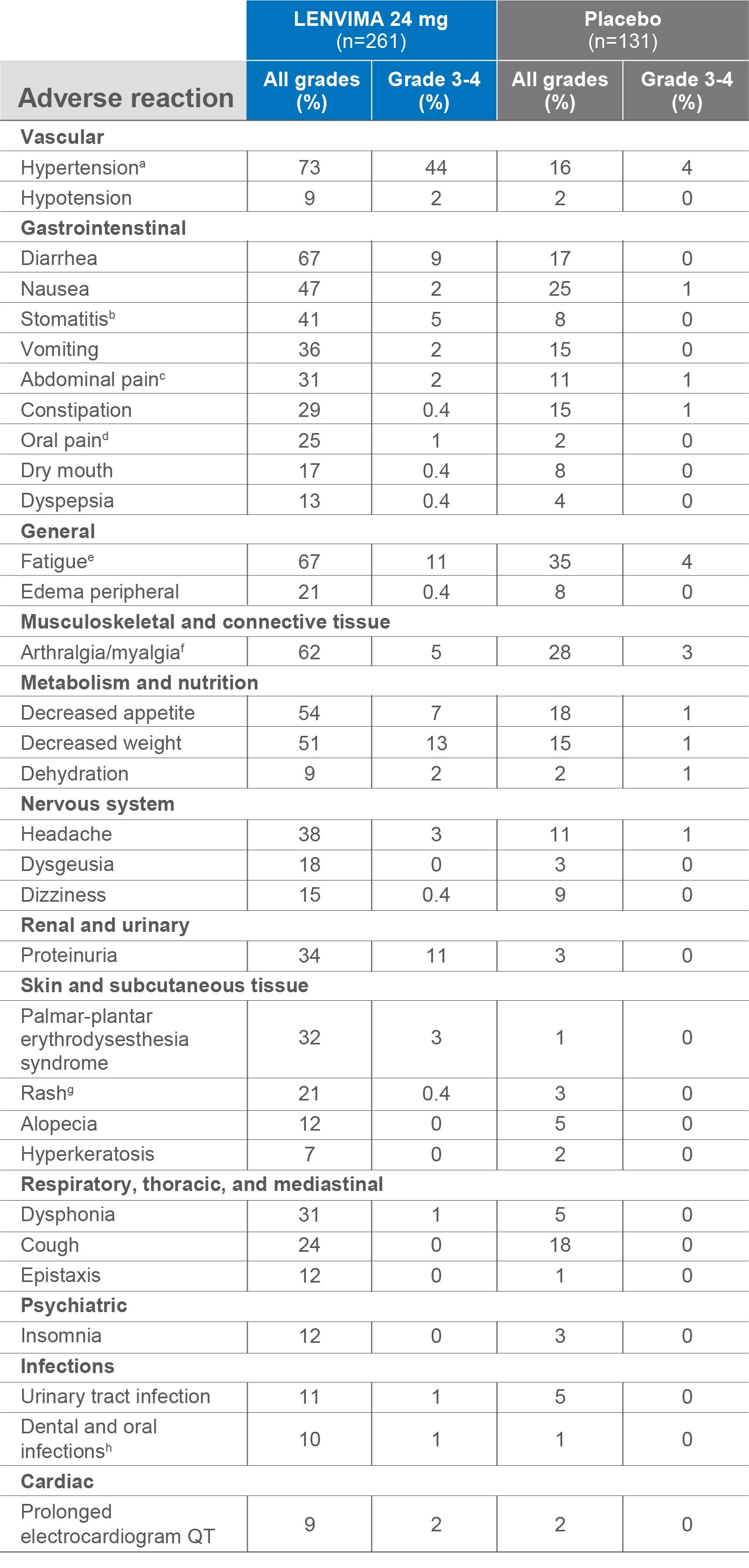
No Grade 4 diarrhea, hand-foot skin reaction, fatigue, or proteinuria were reported.2
- aIncludes hypertension, hypertensive crisis, increased blood pressure diastolic, and increased blood pressure.
- bIncludes aphthous stomatitis, stomatitis, glossitis, mouth ulceration, and mucosal inflammation.
- cIncludes abdominal discomfort, abdominal pain, lower abdominal pain, upper abdominal pain, abdominal tenderness, epigastric discomfort, and gastrointestinal pain.
- dIncludes oral pain, glossodynia, and oropharyngeal pain.
- eIncludes asthenia, fatigue, and malaise.
- fIncludes musculoskeletal pain, back pain, pain in extremity, arthralgia, and myalgia.
- gIncludes macular rash, maculo-papular rash, generalized rash, and rash.
- hIncludes gingivitis, oral infection, parotitis, pericoronitis, penodontitis, sialadenitis, tooth abscess, and tooth infection.
ARs=adverse reactions.
Post hoc analysis of time to first onset of certain ARs with LENVIMA in the SELECT trial3
Identify points in treatment when ARs with LENVIMA emerged in the SELECT trial

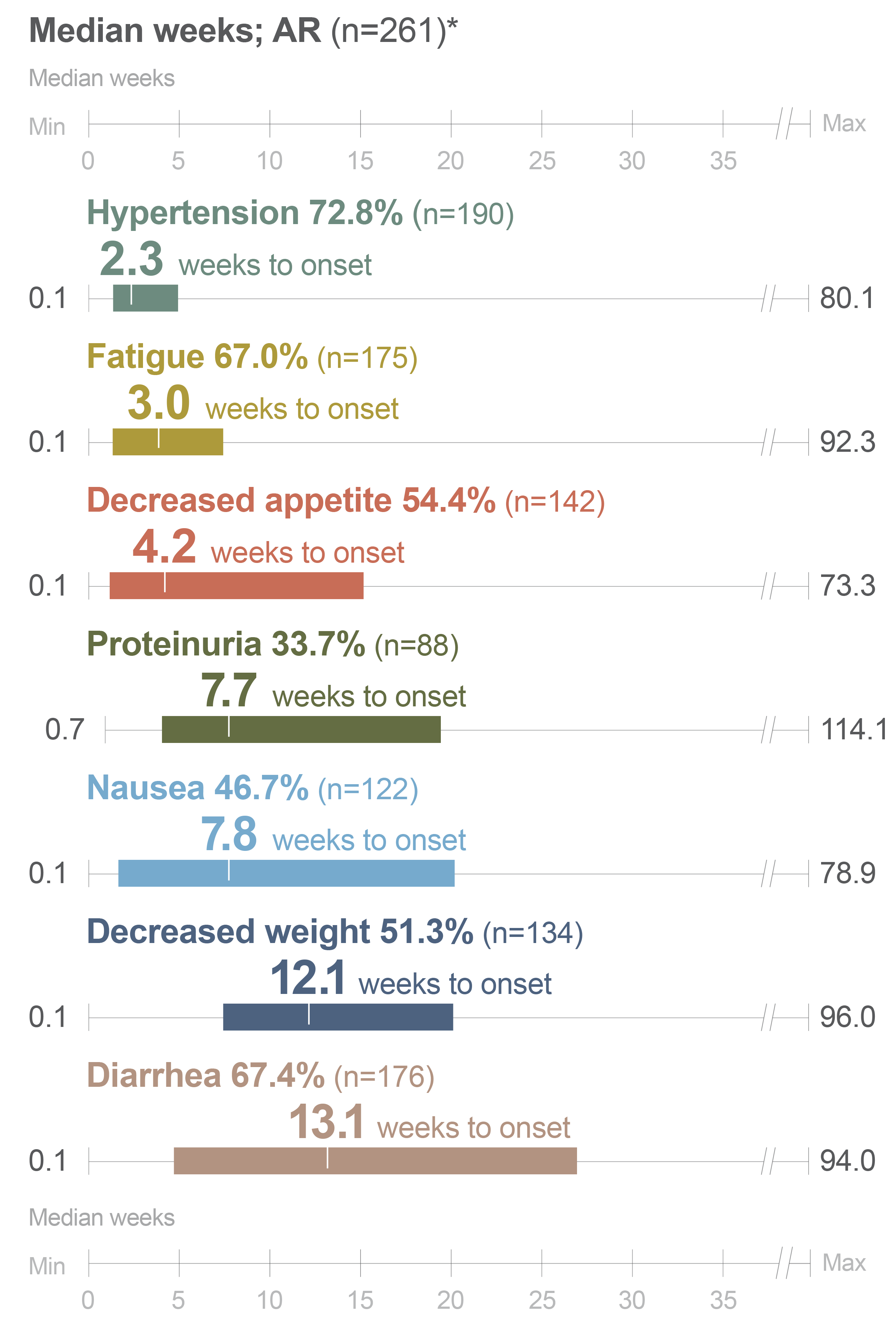
- Monitor your patients for ARs throughout treatment with LENVIMA
Limitation: This is a post hoc exploratory analysis for descriptive purposes only; no conclusion can be drawn.
Please see Table 1 in the full Prescribing Information for Recommended Dosage Modifications for Adverse Reactions.
ARs=adverse reactions; SELECT=Study of (E7080) LEnvatinib in Differentiated Cancer of the Thyroid.
*The bar represents the time to first onset of select ARs for the middle 50% of the patients who experienced that AR from Quartile 1 to 3.
