LENVIMA dosing and administration
Once a day. Every day. With or without food1


Recommended LENVIMA dose: 24 mg (two 10-mg capsules and one 4-mg capsule).1
- Place the required number of capsules, up to a maximum of 5, in a small container (approximately 20 mL capacity) or syringe (20 mL). Do not break or crush capsules
- Add 3 mL of liquid to the container or syringe. Wait 10 minutes for the capsule shell (outer surface) to disintegrate, then stir or shake the mixture for 3 minutes until capsules are fully disintegrated and administer the entire contents
- Next, add an additional 2 mL of liquid to the container or syringe using a second syringe or dropper, swirl or shake and administer. Repeat this step at least once and until there is no visible residue to ensure all of the medication is taken
- If 6 capsules are required for a dose, follow these instructions using 3 capsules at a time
Preparation of suspension:
If LENVIMA suspension is not used at the time of preparation, LENVIMA suspension may be stored in a refrigerator at 36°F to 46°F (2°C to 8°C) for a maximum of 24 hours in a covered container. If not administered within 24 hours, the suspension should be discarded.
Note: Compatibility has been confirmed for polypropylene syringes and for feeding tubes of at least 5 French diameter (polyvinyl chloride or polyurethane tube) and at least 6 French diameter (silicone tube).
Dose modifications for renal or hepatic impairment
Recommended dose of LENVIMA for severe renal or hepatic impairment1
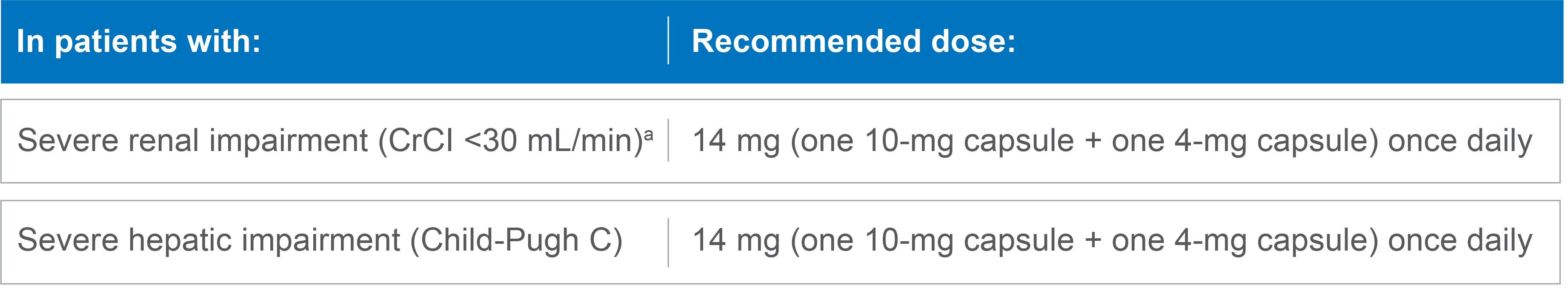
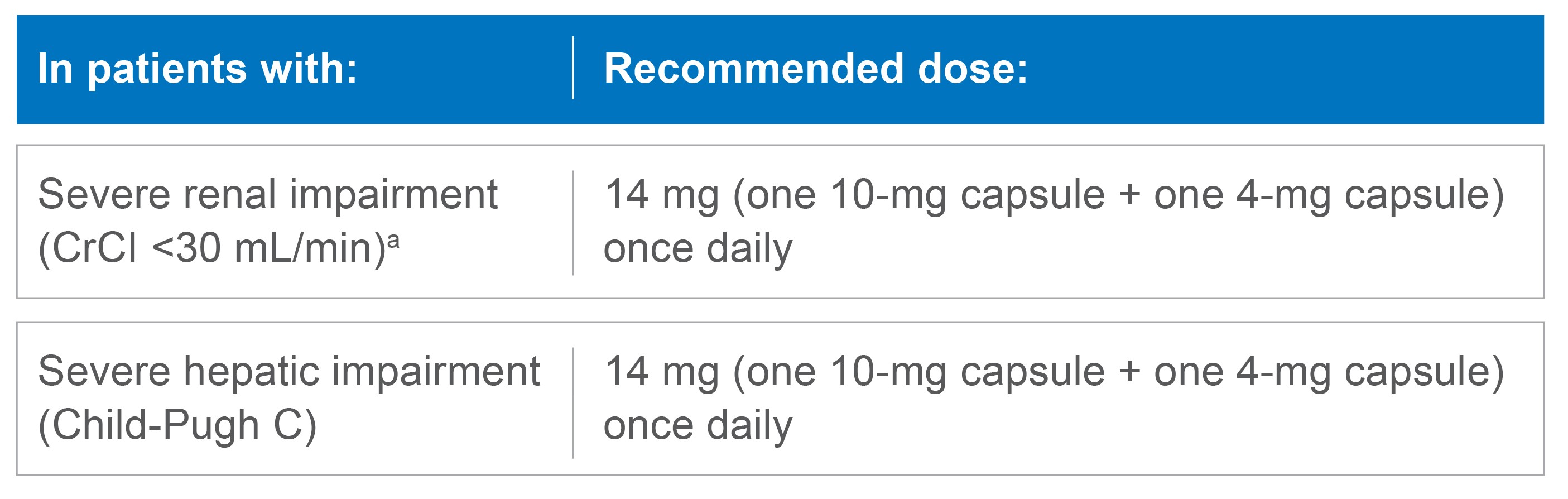
No dose modification is recommended in patients with mild or moderate renal or hepatic impairment.*
LENVIMA has not been studied in patients with end-stage renal disease.
CrCl=creatinine clearance.
- *Mild renal impairment is defined as CrCl 60-89 mL/min and moderate renal impairment is defined as CrCl 30-59 mL/min. Mild and moderate hepatic impairment is defined as Child-Pugh A and Child-Pugh B, respectively.
- aAs calculated by the Cockcroft-Gault equation.
LENVIMA capsules are supplied in cartons of 6 blister cards. Each carton contains a 30-day supply of LENVIMA capsules1
- Continue LENVIMA until disease progression or until unacceptable toxicity
- LENVIMA should be taken at the same time each day. If a patient misses a dose, and it cannot be taken within 12 hours, then that dose should be skipped, and the next dose should be taken at the usual time of administration
- LENVIMA is available as 10-mg and 4-mg capsules



Eisai Engage
Connect to HCP services at Eisai Engage to request materials for your practice, contact a sales representative, and more.
Visit Eisai Engage now